Exhibit 99.1

October 2025 NASDAQ: AVR | ASX: AVR

Disclaimer This presentation has been prepared by Anteris Technologies Global
Corp. (“Anteris,” the “Company,” “we” or “us” or "our"). This presentation, and its contents and the accompanying discussion with management are confidential and may not be further copied, distributed or passed on, directly or indirectly, to
any other person or published or reproduced directly or indirectly, in whole or in part, by any medium or in any form for any purpose without the Company’s prior written consent. The recipient should not construe the contents of this
presentation as legal, tax, accounting, investment advice or recommendation or business, financial or related advice. The recipient should consult its own counsel and tax and financial advisors as to legal and related matters concerning the
matters described in this presentation. This presentation does not purport to be all-inclusive or to contain all of the information that the recipient may require. To the maximum extent permitted by law, none of the Company, its
representatives, nor any other person accepts any liability, including, without limitation, any liability arising out of fault or negligence for any loss arising from the use of the information contained in this presentation. Forward-Looking
Statements This presentation (including oral commentary that accompanies this presentation) contains forward-looking statements, including statements related to our business, products and the PARADIGM Trial. Any statements about our
expectations, beliefs, plans, predictions, forecasts, objectives, assumptions, or future events or performance are not historical facts and may be forward-looking. In some cases, you can identify forward-looking statements through the use of
words such as “believes,” “expects,” “may,” “will,” “should,” “would,” “seeks,” “intends,” “plans,” “pro forma,” “estimates,” “contemplates,” “aims,” “continues,” “anticipates” and similar expressions. Although we believe that the expectations
reflected in these forward-looking statements are reasonable, these statements are not guarantees of future performance and involve risks and uncertainties which are subject to change based on various important factors, some of which are beyond
our control. Among the factors that could cause actual results to differ materially from those suggested by forward-looking statements are: our current and future research and development activities, including clinical testing and manufacturing
and related costs and timing; sufficiency of our capital resources; our product development and business strategy, including the potential size of the markets for our products and future development and/or expansion of our products in our
markets; our ability to commercialize products and generate product revenues; our ability to raise additional funding when needed; any statements concerning anticipated regulatory activities, including our ability to obtain regulatory
clearances; our research and development expenses; and risks facing our operations and intellectual property; and the other risks described in our Annual Report on Form 10-K for the year ended December 31, 2024 and the other filings we make
with the Securities and Exchange Commission. Should one or more of these risks or uncertainties materialize or should any of these assumptions prove to be incorrect, our actual future results, performance and events and circumstances may differ
in material respects from the performance projected in these forward-looking statements. The forward-looking statements included in this presentation are made only as of the date hereof. The Company does not undertake any obligation to update
any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in the Company’s expectations, except as may be required by law. Accordingly, the Company cautions you
not to place any undue reliance on any forward-looking statements. Industry Data This presentation also includes data, forecasts and information obtained from industry publications and other information available to us. Some data is also
based on our good faith estimates, which are derived from management’s knowledge of the industry and independent sources. We have not independently verified any of the data from third-party sources, nor have we ascertained the underlying
assumptions relied upon therein. While we are not aware of any misstatements regarding the industry data presented herein, estimates and forecasts involve uncertainties and risks and are subject to change based on various
factors. Milestones This presentation contains various milestones. These milestones are not projections and instead are forward-looking goals that are subject to significant business, economic, regulatory and competitive uncertainties and
contingencies, many of which are beyond the control of the Company and its management and are based upon assumptions with respect to future decisions, which are subject to change. Actual results will vary, and those variations may be material.
Nothing in this presentation should be regarded as a representation by any person that these milestones will be achieved and the Company undertakes no duty to update these milestones. No Offer or Solicitation This presentation shall not
constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful. Before you invest, you
should read the documents we file with the SEC for more complete information about us. You can obtain these documents for free by visiting EDGAR on the SEC’s website at www.sec.gov. 2

3

Anteris Technologies – Executing on strategy 4 Building commercial readiness
for a new class of TAVR that mimics a healthy aortic valve Successfully priced U.S. IPO and listed on Nasdaq in December 2024 - Enhancing liquidity and visibility in the world’s largest healthcare investment market 1 Total of 130 DurAVR®
THV patients, 49 patients treated YTD- Building momentum, 38% of all patients enrolled in 6 months (Jan-Jun 2025) 2 Data showcased by global KOLs at leading cardiovascular conferences- CRT (Mar), Sydney Valves (Mar), Euro PCR (May), CSI
Frankfurt (Jun), New York Valves (Jun) 3 First-in-human DUAL valve-in-valve success with DurAVR® THV- DurAVR® successfully implanted in both aortic (Mar) and mitral ViV procedures (May) 4 Hosted global investigator meeting to launch
pivotal PARADIGM Trial- Setting the foundation for accelerated site activation and patient enrollment (Jun) 5

Investment Highlights 5 The only biomimetic balloon expandable TAVR with
130 treated patients Path to Commercialization Poised to disrupt a high value, growth market DurAVR® THV is the only balloon expandable aortic valve to deliver curative, pre-disease hemodynamics1,2. Forecasted US$9.9bn by 2028 (US12.5bn
with Valve-in-Valve)3. Underpenetrated with 80-85% of severe aortic stenosis patients untreated4. 130 patients. Strong performance at 30 days & 1 year. PARADIGM Pivotal trial targeted 4Q25*, 50% DurAVR® vs. 50% SAPIEN or Evolut.
Potential pathway to FDA & CE Mark approval. Scaled manufacturing, engaged global KOLs, early adopter site identification. Compact market allows a capital-efficient launch with lean, scalable field force. Established TAVR reimbursement
pathways. Proprietary, First-in-Class TAVR Multi-billion-dollar global TAVR market Clinical Validation Commercial Readiness Commercial Launch Garg, P., Markl, M., Sathananthan, J. et al. Restoration of flow in the aorta: a novel
therapeutic target in aortic valve intervention. Nat Rev Cardiol 21, 264–273 (2024). https://doi.org/10.1038/s41569-023-00943-6. Garg, P. DurAVR® TAVI: biomimetic design restores flow and leads to significant LV mass regression. MRI study.
Oral presentation at PCR London Valves; Nov 2024; London, England. Future Market Insights. Transcatheter Heart Valve Replacement (TAVR) Market: Global Industry Analysis 2016 – 2023 and Opportunity Assessment 2024 – 2034. Future Market
Insights; 2024. Available from: https://www.futuremarketinsights.com/reports/transcatheter-heart-valve-replacement-tavi-market Gahl B, Çelik M, Head SJ, et al. Natural History of Asymptomatic Severe Aortic Stenosis and the Association of
Early Intervention With Outcomes: A Systematic Review and Meta-analysis. JAMA Cardiol. 2020;5(10):1102–1112. doi:10.1001/jamacardio.2020.2497. *Subject to regulatory approval

Highly Experienced Leadership – Clinical, Operational, Commercial 6 Mr.
McDonnell has served as CFO since November 2018. Prior to his appointment he worked at KPMG for over 24 years, where Mr. McDonnell held several senior positions including 10 years as a partner. He has experience in restructurings, acquisitions,
divestments, privatizations and other significant financial transactions. Matthew McDonnell Chief financial officer Dr. Meduri has served as Anteris’ CMO since August 2021. Dr. Meduri is a practicing Interventional Cardiologist at Stern
Cardiovascular Foundation, Memphis, TN and a recognized global leader in the field of valvular heart disease with over 3,500 career structural heart procedures and over 300 annually. He has served as global head of many TAVR, mitral and
tricuspid trials. Dr. Chris Meduri Chief medical officer Mr. Paterson has served as CEO since March 2017 and was appointed Vice Chairman in March 2025. He held global positions in big pharma incl. Merck KGaA (“Merck”) from 2005-2013, and
Roche (1995-2005). His roles included Global Head of CV Medicine, President of Europe, Israel, Canada & Australia, President of Emerging Markets incl. Russia & LATAM, CEO of Japan, Head of Commercial Operations in China, Head of Korea,
and Product Manager. He also sat on a NASDAQ board (CHPD) and led a $5B sale of that business. He has launched global healthcare products 36 times totaling billions in revenue and driven dozens of acquisitions, in-licensing and out-licensing
deals globally. Wayne Paterson VICE CHAIRMAN & CEO Mr. St Denis has served as COO since July 2017 and was appointed President and Director in March 2025. From 2008-2017 he held senior positions at Merck including Head of Commercial
Operations for Europe and Canada, and Head of Operations for Emerging Markets. From 1996-2006, he held senior roles at Millennium Pharmaceuticals Inc. David St Denis President & Director

Board of Directors 7 John Seaberg Chairman Mr. Seaberg has been Chairman since
March 2017 and a director since October 2014. He has served as Board Chair of Preceptis Medical Inc since 2016 and Phraxis Medical Inc since 2009. He was Executive VP at Cedar Point Capital from 2015-2023. He was Chair of Synovis Inc., a
manufacturer of medical devices and tissue products from 2008-2012. Mr. Paterson has served as CEO since March 2017 and was appointed Vice Chairman in March 2025. He held global positions in big pharma incl. Merck KGaA (“Merck”) from
2005-2013, and Roche (1995-2005). His roles included Global Head of CV Medicine, President of Europe, Israel, Canada & Australia, President of Emerging markets incl. Russia & LATAM, CEO of Japan, Head of Commercial Ops in China. He sat
on a NASDAQ board (CHPD) and led a $5B sale of that business. He has launched 36 global healthcare products totaling billions in revenue. Wayne Paterson VICE CHAIRMAN & CEO DIRECTOR & Company secretary Mr. St Denis has served as COO
since July 2017 and was appointed President and Director in March 2025. From 2008-2017 he held senior positions at Merck including Head of Commercial Operations for Europe and Canada, and Head of Operations for Emerging Markets. From 1996-2006,
he held senior roles at Millennium Pharmaceuticals Inc. David St Denis President & Director Greg Moss Non-Executive Director Mr. Moss serves as Chief Business and Legal Officer, as well as Corporate Secretary and Chief Compliance
Officer of Evommune, Inc. Prior to Evommune, he served as Executive Vice President, General Counsel, and Corporate Secretary, Chief Compliance Officer at Kadmon, culminating in Kadmon’s $1.9 billion acquisition in 2021. Dave
Roberts Non-Executive Director Mr. Roberts joined LeMaitre Vascular (NASDAQ: LMAT) in 1997 as its twelfth employee and has served as a Board Director since 2001 and as President since 2007. Mr. Roberts has also served as a Board Director of
Lexington Medical since 2023 and of Parasole Restaurant Holdings since 2013. Stephen Denaro Mr. Denaro has been a director and Company Secretary since October 2018. Mr. Denaro serves as director and sole shareholder of Trio Business
Intermediaries Pty Ltd. He has over 25 years of experience in mergers and acquisitions, business valuations, accountancy services, and income tax compliance.

Global Manufacturing Footprint 8 Purpose-built infrastructure designed for
efficient scale-up and commercial readiness
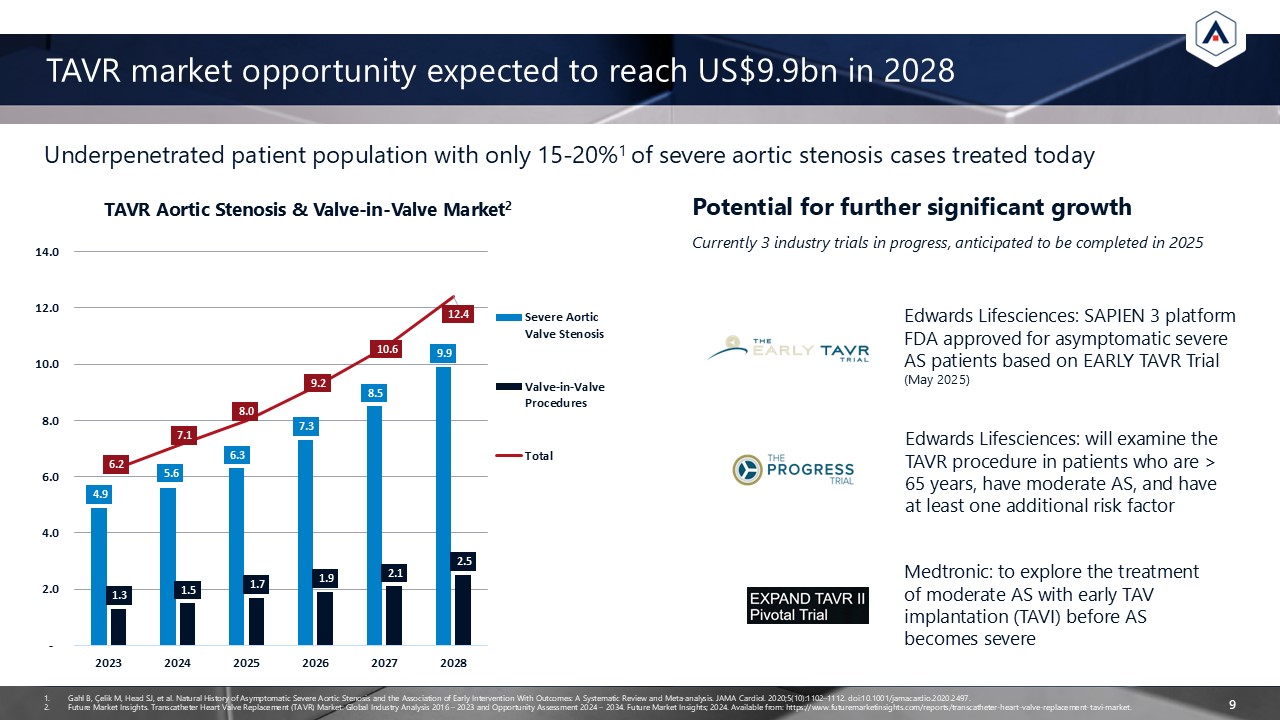
TAVR market opportunity expected to reach US$9.9bn in 2028 9 Currently 3
industry trials in progress, anticipated to be completed in 2025 Potential for further significant growth Edwards Lifesciences: SAPIEN 3 platform FDA approved for asymptomatic severe AS patients based on EARLY TAVR Trial (May
2025) Medtronic: to explore the treatment of moderate AS with early TAV implantation (TAVI) before AS becomes severe Edwards Lifesciences: will examine the TAVR procedure in patients who are > 65 years, have moderate AS, and have at least
one additional risk factor Gahl B, Çelik M, Head SJ, et al. Natural History of Asymptomatic Severe Aortic Stenosis and the Association of Early Intervention With Outcomes: A Systematic Review and Meta-analysis. JAMA Cardiol.
2020;5(10):1102–1112. doi:10.1001/jamacardio.2020.2497. Future Market Insights. Transcatheter Heart Valve Replacement (TAVR) Market: Global Industry Analysis 2016 – 2023 and Opportunity Assessment 2024 – 2034. Future Market Insights; 2024.
Available from: https://www.futuremarketinsights.com/reports/transcatheter-heart-valve-replacement-tavi-market. Underpenetrated patient population with only 15-20%1 of severe aortic stenosis cases treated today
A New Class of TAVR
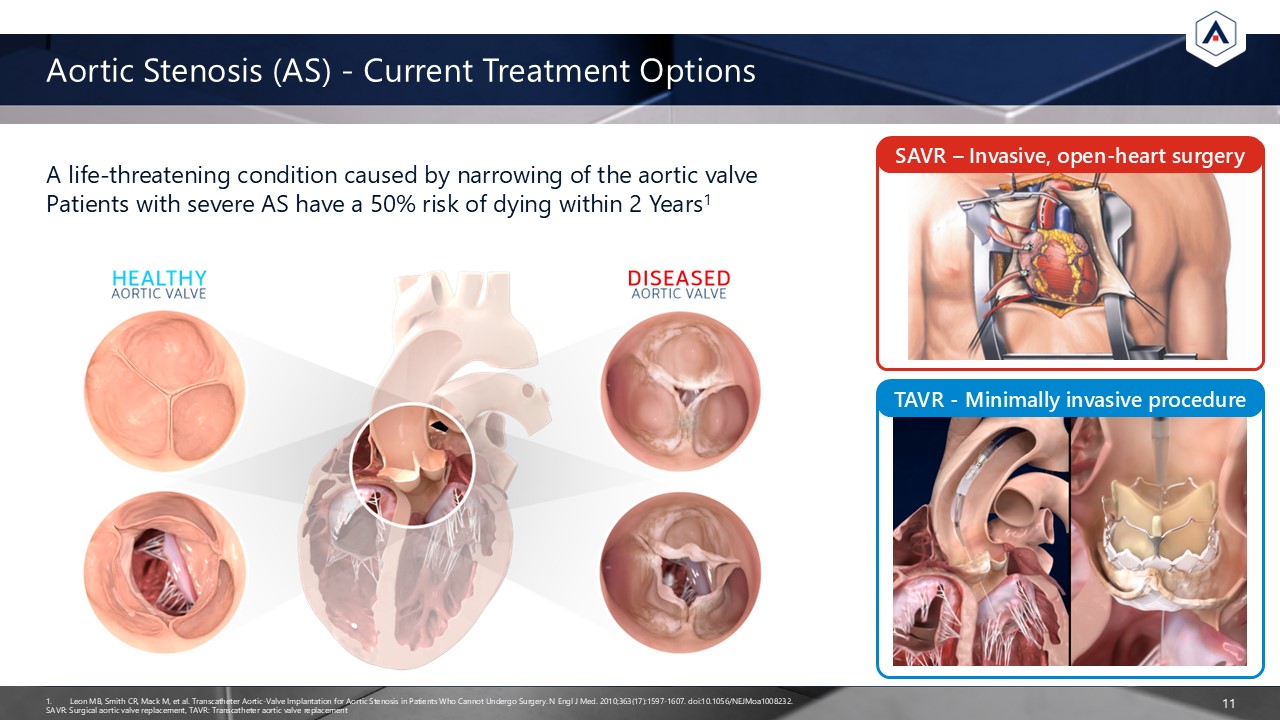
Aortic Stenosis (AS) - Current Treatment Options 11 A life-threatening
condition caused by narrowing of the aortic valvePatients with severe AS have a 50% risk of dying within 2 Years1 Leon MB, Smith CR, Mack M, et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo
Surgery. N Engl J Med. 2010;363(17):1597-1607. doi:10.1056/NEJMoa1008232. SAVR: Surgical aortic valve replacement, TAVR: Transcatheter aortic valve replacement SAVR – Invasive, open-heart surgery TAVR - Minimally invasive procedure
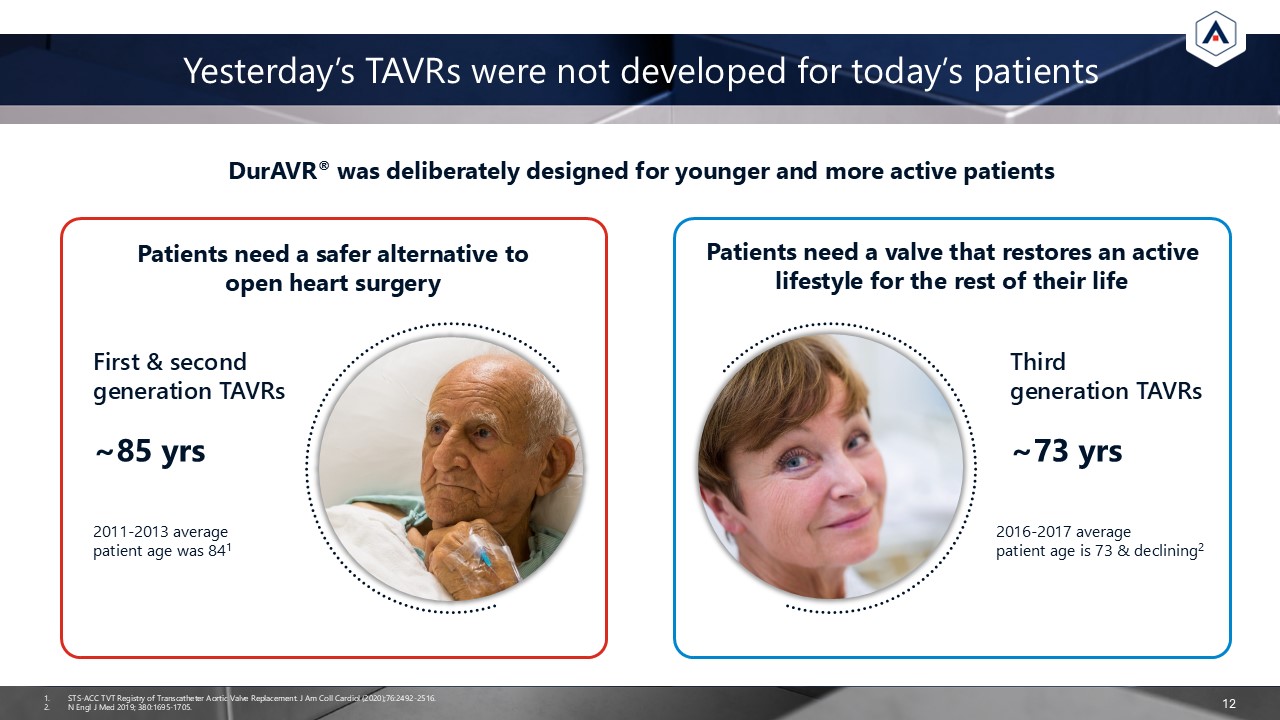
12 Yesterday’s TAVRs were not developed for today’s patients DurAVR® was
deliberately designed for younger and more active patients STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol (2020);76:2492-2516. N Engl J Med 2019; 380:1695-1705. Patients need a safer alternative to open
heart surgery Patients need a valve that restores an active lifestyle for the rest of their life First & second generation TAVRs ~85 yrs Third generation TAVRs ~73 yrs 2011-2013 average patient age was 841 2016-2017 average patient
age is 73 & declining2
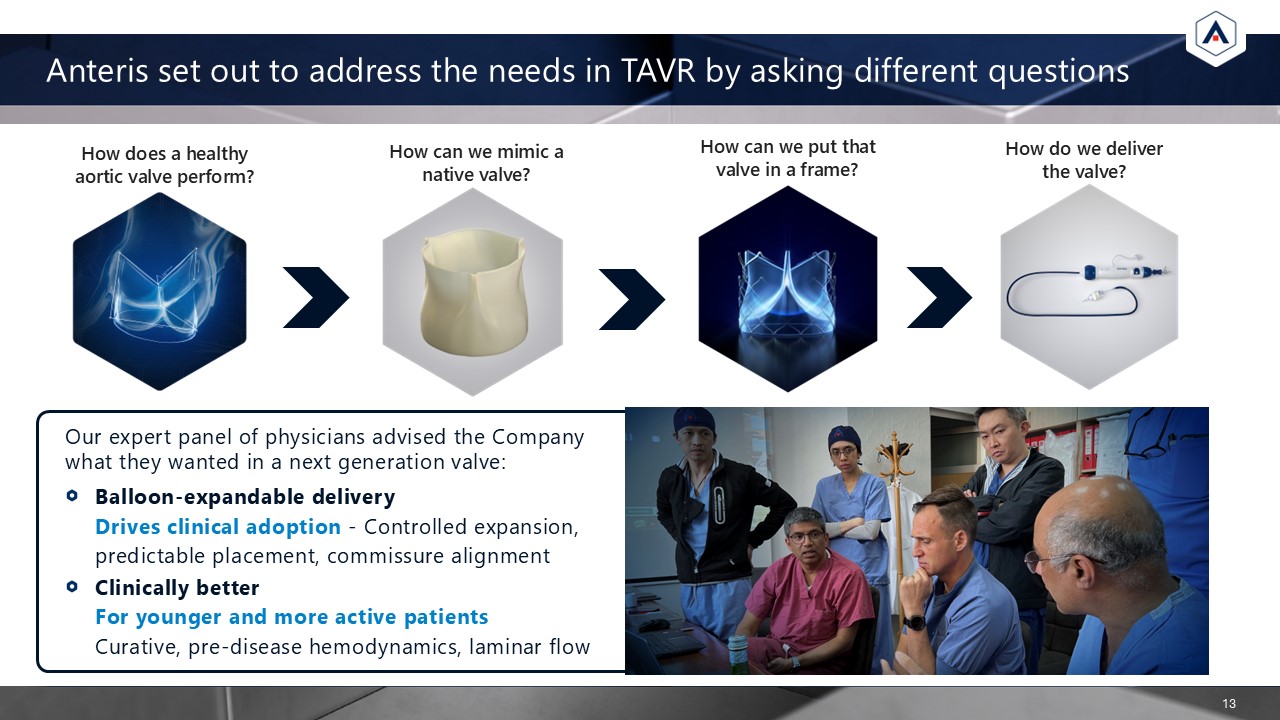
Anteris set out to address the needs in TAVR by asking different
questions 13 Our expert panel of physicians advised the Company what they wanted in a next generation valve: Balloon-expandable deliveryDrives clinical adoption - Controlled expansion, predictable placement, commissure alignment Clinically
better For younger and more active patients Curative, pre-disease hemodynamics, laminar flow How can we mimic a native valve? How does a healthy aortic valve perform? How do we deliver the valve? How can we put that valve in a frame?
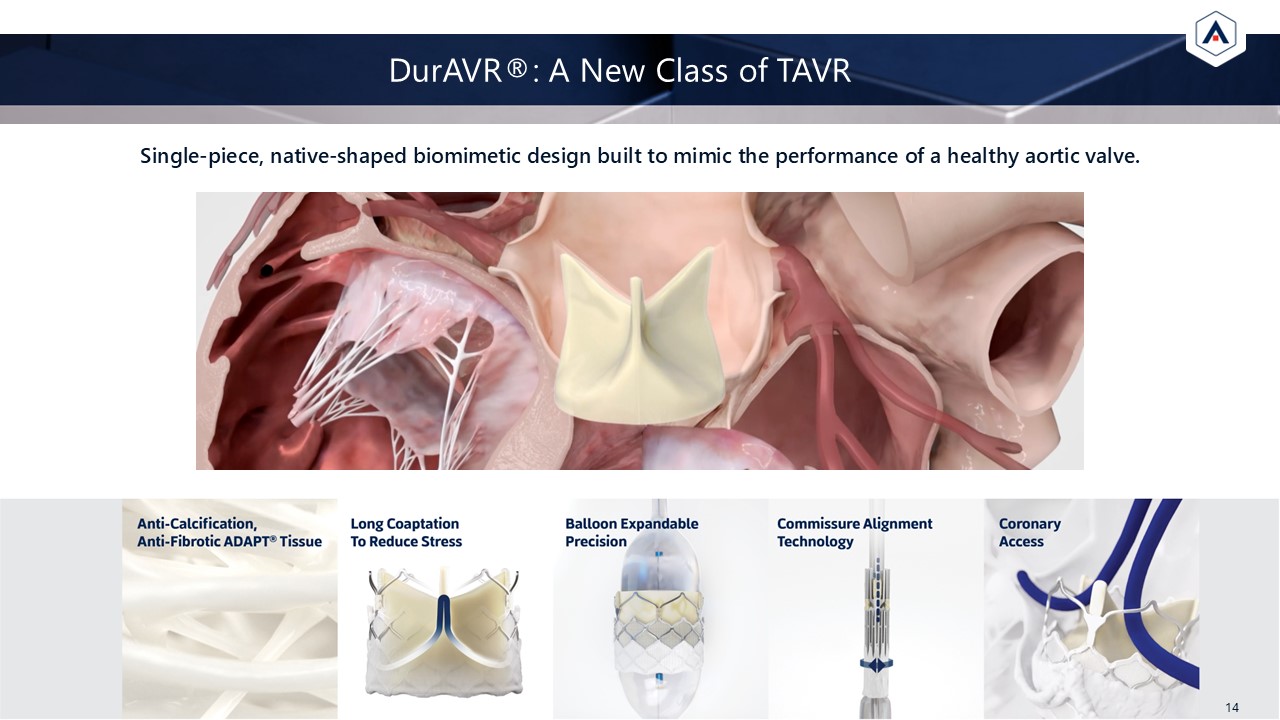
Single-piece, native-shaped biomimetic design built to mimic the performance of a
healthy aortic valve. DurAVR®: A New Class of TAVR 14
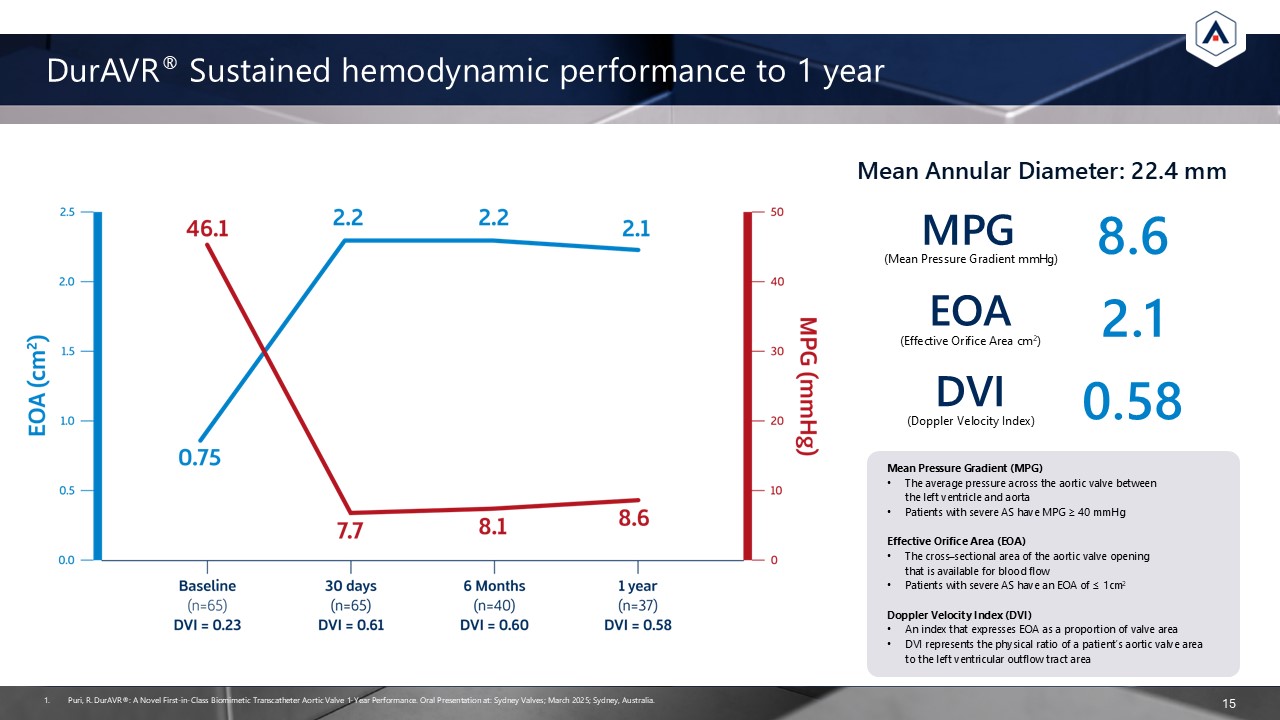
DurAVR® Sustained hemodynamic performance to 1 year 15 Puri, R. DurAVR®: A Novel
First-in-Class Biomimetic Transcatheter Aortic Valve 1-Year Performance. Oral Presentation at: Sydney Valves; March 2025; Sydney, Australia. MPG (Mean Pressure Gradient mmHg) DVI (Doppler Velocity Index) 2.1 EOA (Effective Orifice Area
cm2) 8.6 0.58 Mean Annular Diameter: 22.4 mm Mean Pressure Gradient (MPG) The average pressure across the aortic valve between the left ventricle and aorta Patients with severe AS have MPG ≥ 40 mmHg Effective Orifice Area (EOA) The
cross–sectional area of the aortic valve opening that is available for blood flow Patients with severe AS have an EOA of ≤ 1cm2 Doppler Velocity Index (DVI) An index that expresses EOA as a proportion of valve area DVI represents the
physical ratio of a patient’s aortic valve area to the left ventricular outflow tract area
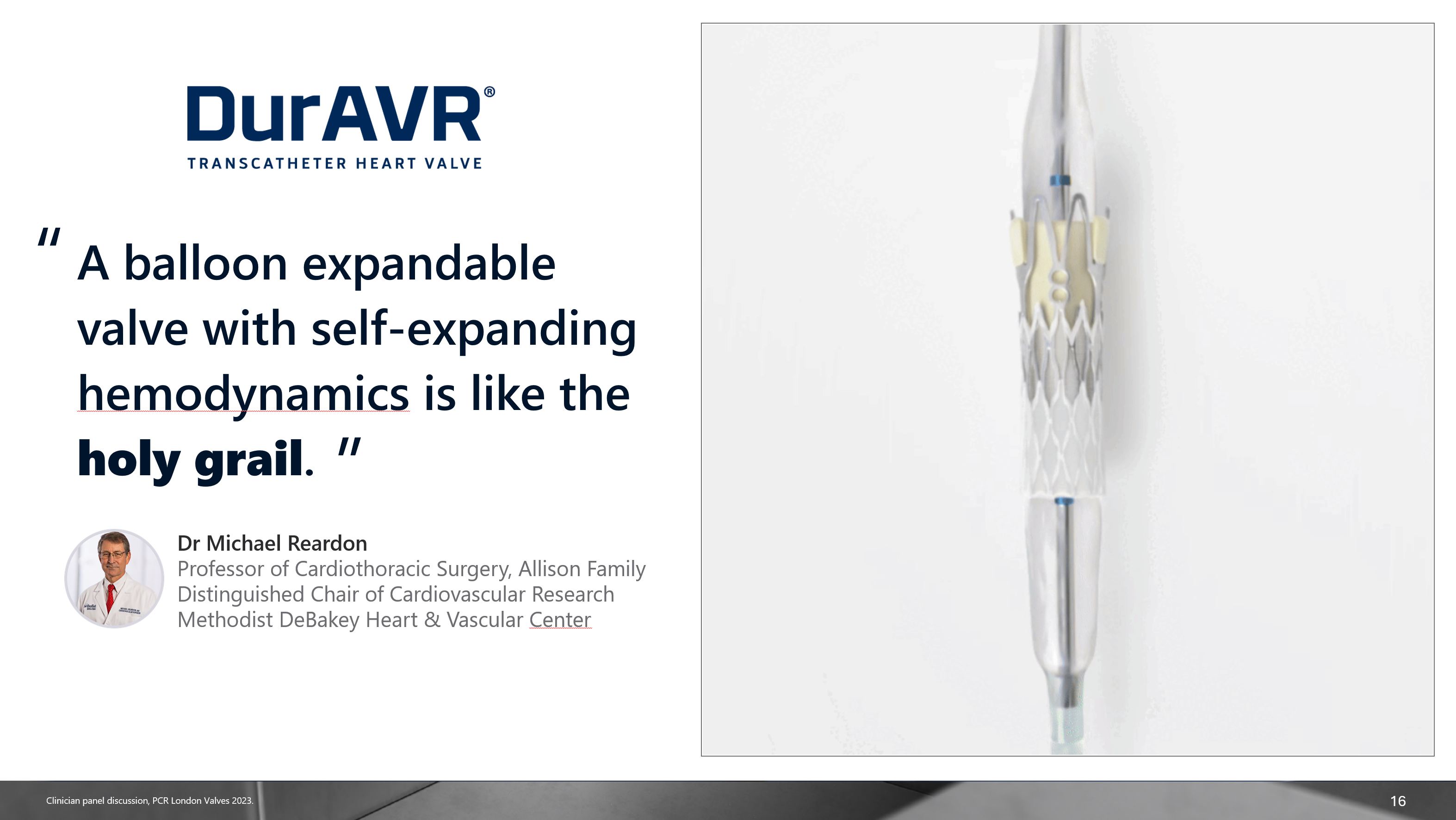
16 “ A balloon expandable valve with self-expanding hemodynamics is like the
holy grail. Dr Michael ReardonProfessor of Cardiothoracic Surgery, Allison Family Distinguished Chair of Cardiovascular Research Methodist DeBakey Heart & Vascular Center “ Clinician panel discussion, PCR London Valves 2023.
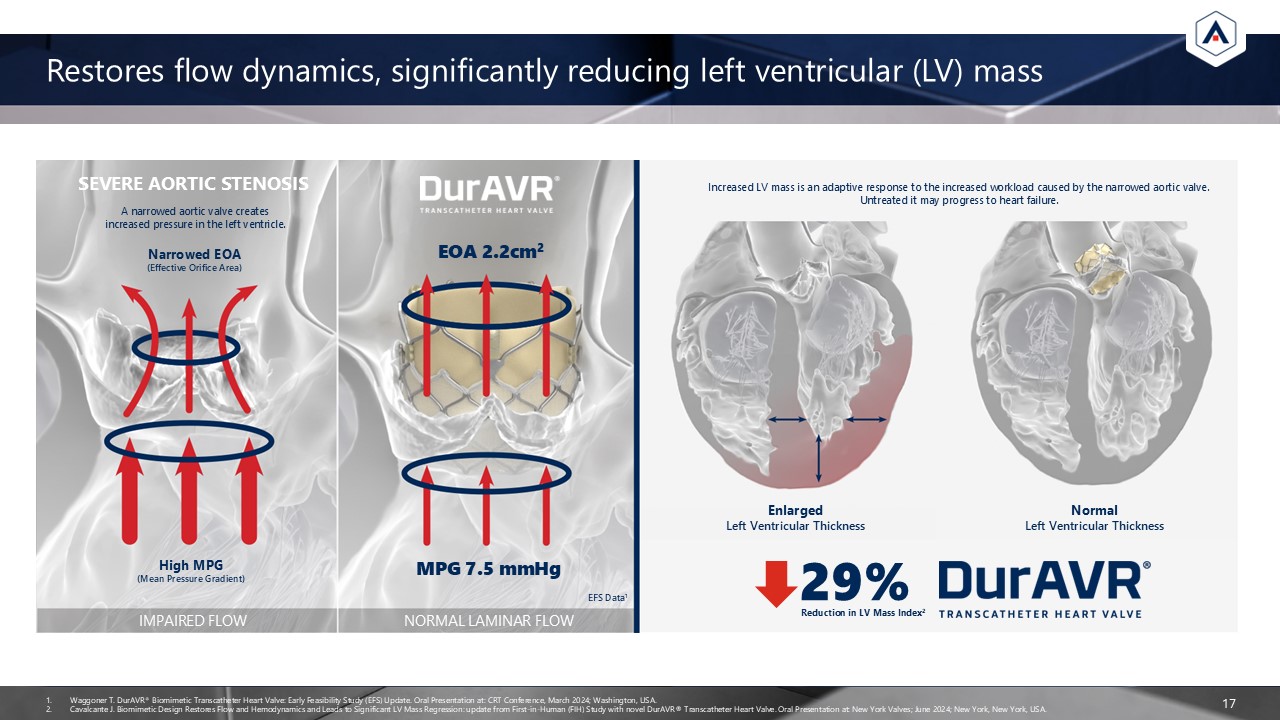
Restores flow dynamics, significantly reducing left ventricular (LV)
mass 17 SEVERE AORTIC STENOSIS EOA 2.2cm2 MPG 7.5 mmHg IMPAIRED FLOW NORMAL LAMINAR FLOW Waggoner T. DurAVR® Biomimetic Transcatheter Heart Valve: Early Feasibility Study (EFS) Update. Oral Presentation at: CRT Conference, March 2024;
Washington, USA. Cavalcante J. Biomimetic Design Restores Flow and Hemodynamics and Leads to Significant LV Mass Regression: update from First-in-Human (FIH) Study with novel DurAVR® Transcatheter Heart Valve. Oral Presentation at: New York
Valves; June 2024; New York, New York, USA. A narrowed aortic valve creates increased pressure in the left ventricle. Narrowed EOA(Effective Orifice Area) High MPG(Mean Pressure Gradient) EnlargedLeft Ventricular Thickness NormalLeft
Ventricular Thickness Increased LV mass is an adaptive response to the increased workload caused by the narrowed aortic valve. Untreated it may progress to heart failure. EFS Data1 29% Reduction in LV Mass Index2
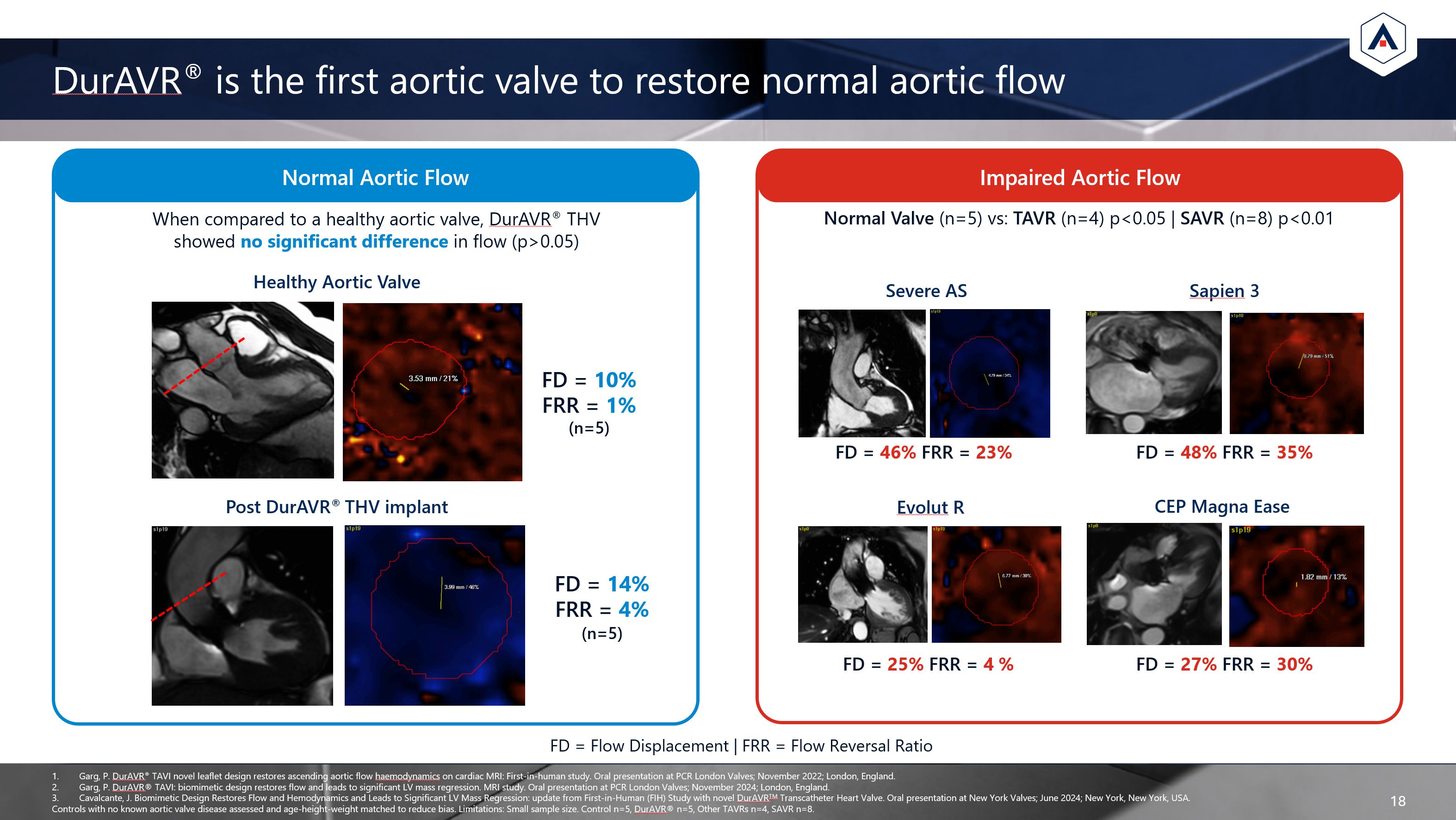
Normal Aortic Flow DurAVR® is the first aortic valve to restore normal aortic
flow 18 Post DurAVR® THV implant Healthy Aortic Valve FD = 14% FRR = 4% (n=5) FD = 46% FRR = 23% When compared to a healthy aortic valve, DurAVR® THV showed no significant difference in flow (p>0.05) Severe AS Sapien 3 CEP
Magna Ease Evolut R FD = 10% FRR = 1% (n=5) FD = 48% FRR = 35% FD = 27% FRR = 30% FD = 25% FRR = 4 % FD = Flow Displacement | FRR = Flow Reversal Ratio Garg, P. DurAVR® TAVI novel leaflet design restores ascending aortic flow
haemodynamics on cardiac MRI: First-in-human study. Oral presentation at PCR London Valves; November 2022; London, England. Garg, P. DurAVR® TAVI: biomimetic design restores flow and leads to significant LV mass regression. MRI study. Oral
presentation at PCR London Valves; November 2024; London, England. Cavalcante, J. Biomimetic Design Restores Flow and Hemodynamics and Leads to Significant LV Mass Regression: update from First-in-Human (FIH) Study with novel DurAVRTM
Transcatheter Heart Valve. Oral presentation at New York Valves; June 2024; New York, New York, USA. Controls with no known aortic valve disease assessed and age-height-weight matched to reduce bias. Limitations: Small sample size. Control
n=5, DurAVR® n=5, Other TAVRs n=4, SAVR n=8. Impaired Aortic Flow Normal Valve (n=5) vs: TAVR (n=4) p<0.05 | SAVR (n=8) p<0.01
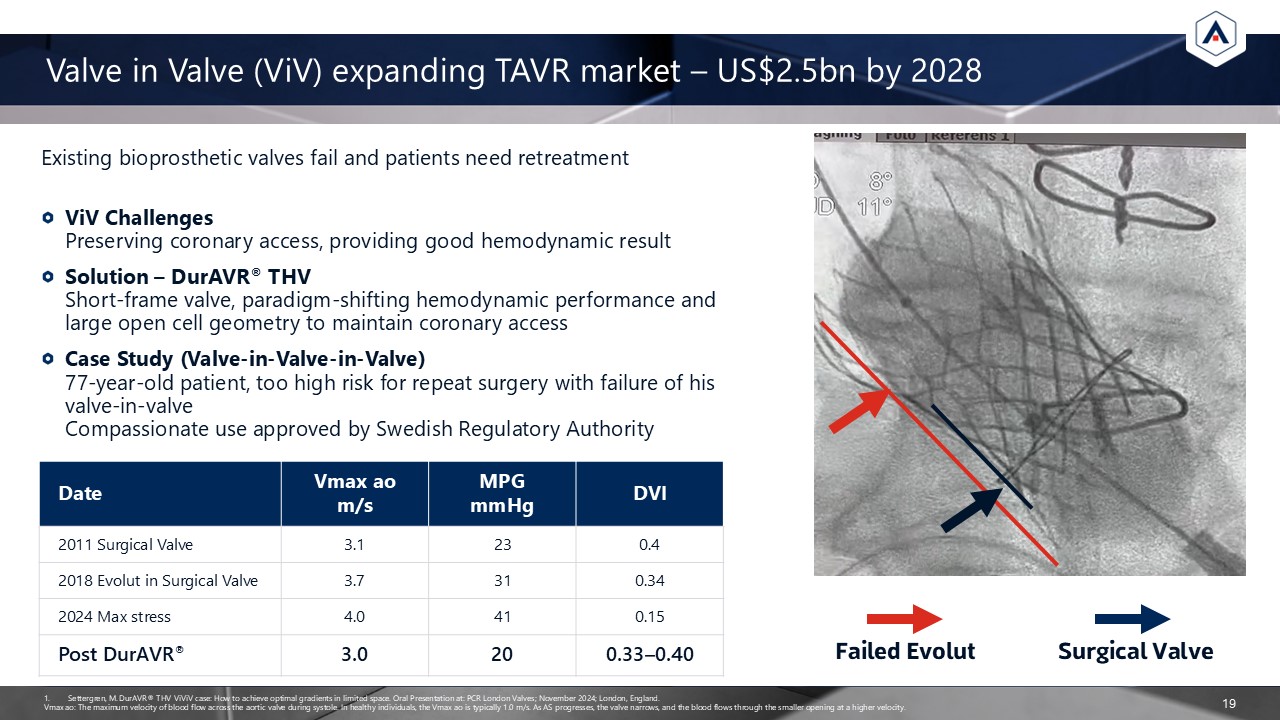
Existing bioprosthetic valves fail and patients need retreatment ViV
ChallengesPreserving coronary access, providing good hemodynamic result Solution – DurAVR® THVShort-frame valve, paradigm-shifting hemodynamic performance and large open cell geometry to maintain coronary access Case Study
(Valve-in-Valve-in-Valve)77-year-old patient, too high risk for repeat surgery with failure of his valve-in-valveCompassionate use approved by Swedish Regulatory Authority Valve in Valve (ViV) expanding TAVR market – US$2.5bn by
2028 19 Date Vmax aom/s MPG mmHg DVI 2011 Surgical Valve 3.1 23 0.4 2018 Evolut in Surgical Valve 3.7 31 0.34 2024 Max stress 4.0 41 0.15 Post DurAVR® 3.0 20 0.33–0.40 Failed Evolut Surgical Valve Settergren, M. DurAVR®
THV ViViV case: How to achieve optimal gradients in limited space. Oral Presentation at: PCR London Valves; November 2024; London, England. Vmax ao: The maximum velocity of blood flow across the aortic valve during systole. In healthy
individuals, the Vmax ao is typically 1.0 m/s. As AS progresses, the valve narrows, and the blood flows through the smaller opening at a higher velocity.

Path to Commercialization
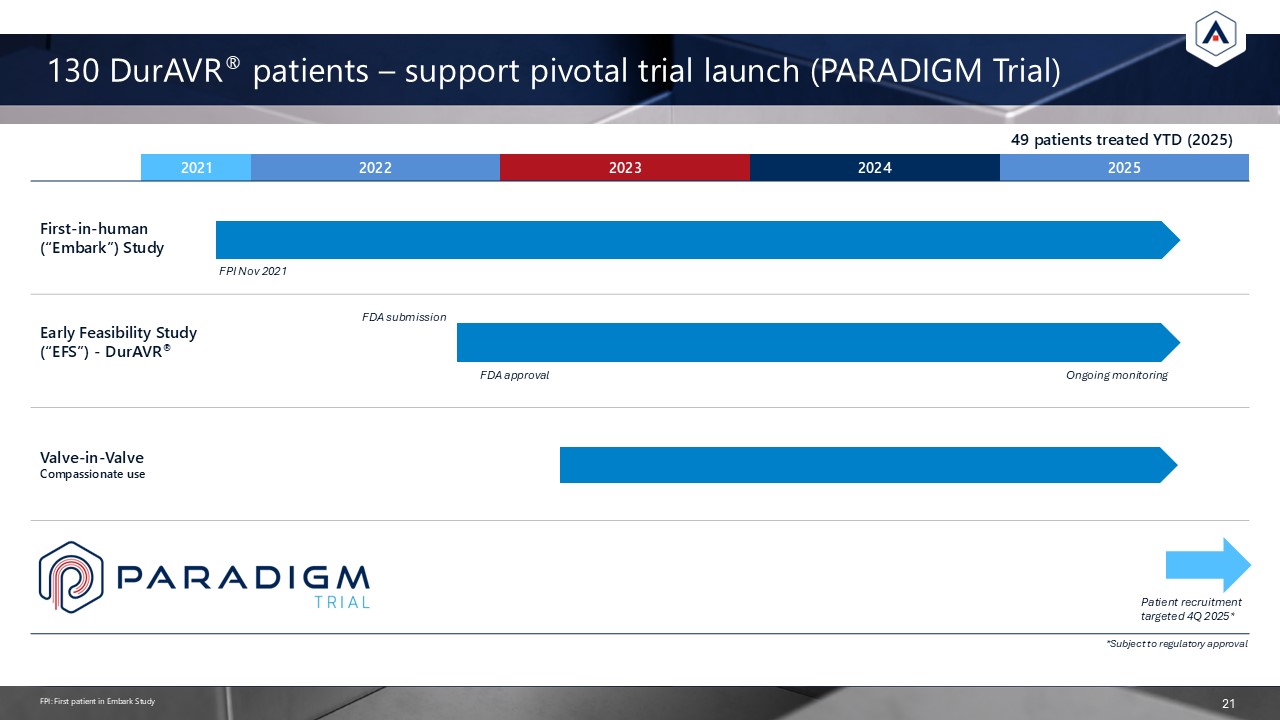
130 DurAVR® patients – support pivotal trial launch (PARADIGM Trial)
21 2021 2022 2023 2024 2025 First-in-human (“Embark”) Study Early Feasibility Study (“EFS”) - DurAVR® Valve-in-Valve Compassionate use *Subject to regulatory approval Patient recruitment targeted 4Q 2025* FDA submission FDA
approval Ongoing monitoring FPI Nov 2021 FPI: First patient in Embark Study 49 patients treated YTD (2025)

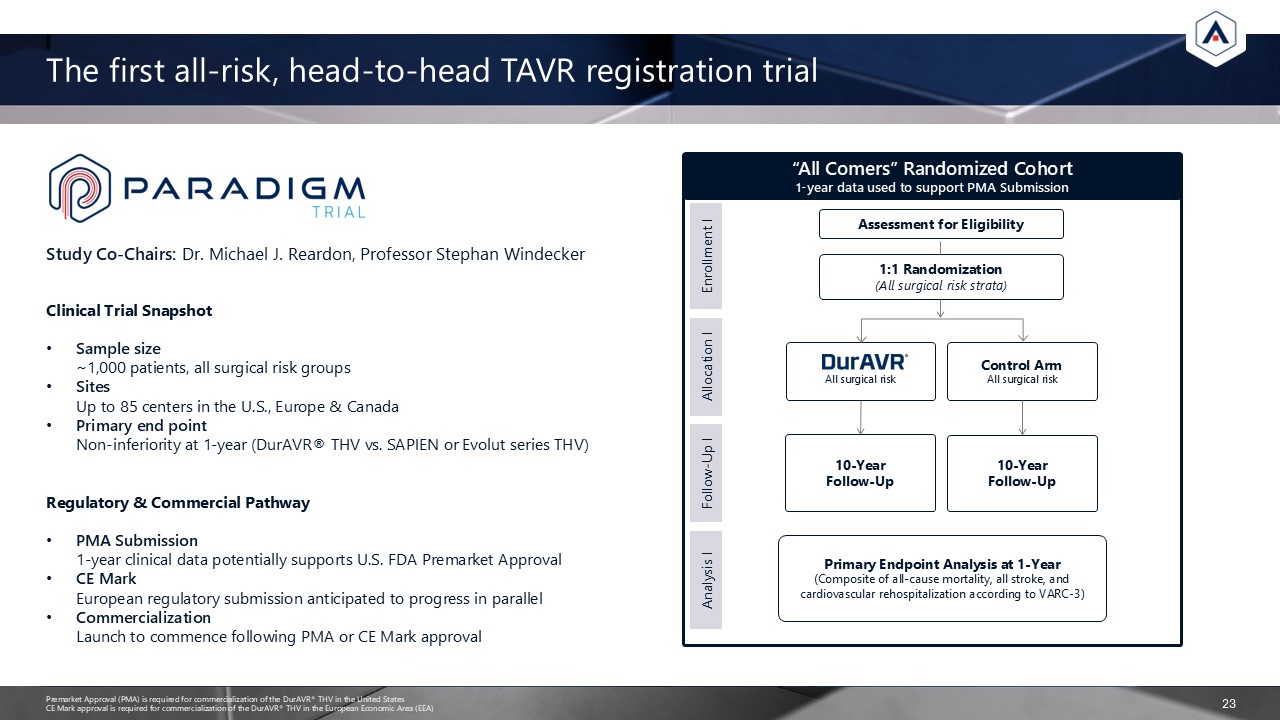
Study Co-Chairs: Dr. Michael J. Reardon, Professor Stephan Windecker The first
all-risk, head-to-head TAVR registration trial “All Comers” Randomized Cohort 1-year data used to support PMA Submission Enrollment I Allocation I Follow-Up I Analysis I Assessment for Eligibility 1:1 Randomization(All surgical risk
strata) All surgical risk Control ArmAll surgical risk 10-YearFollow-Up 10-YearFollow-Up Primary Endpoint Analysis at 1-Year (Composite of all-cause mortality, all stroke, and cardiovascular rehospitalization according to
VARC-3) 23 Clinical Trial Snapshot Sample size ~1,000 patients, all surgical risk groups Sites Up to 85 centers in the U.S., Europe & Canada Primary end pointNon-inferiority at 1-year (DurAVR® THV vs. SAPIEN or Evolut series
THV) Regulatory & Commercial Pathway PMA Submission 1-year clinical data potentially supports U.S. FDA Premarket Approval CE Mark European regulatory submission anticipated to progress in parallel Commercialization Launch to commence
following PMA or CE Mark approval Premarket Approval (PMA) is required for commercialization of the DurAVR® THV in the United StatesCE Mark approval is required for commercialization of the DurAVR® THV in the European Economic Area (EEA)
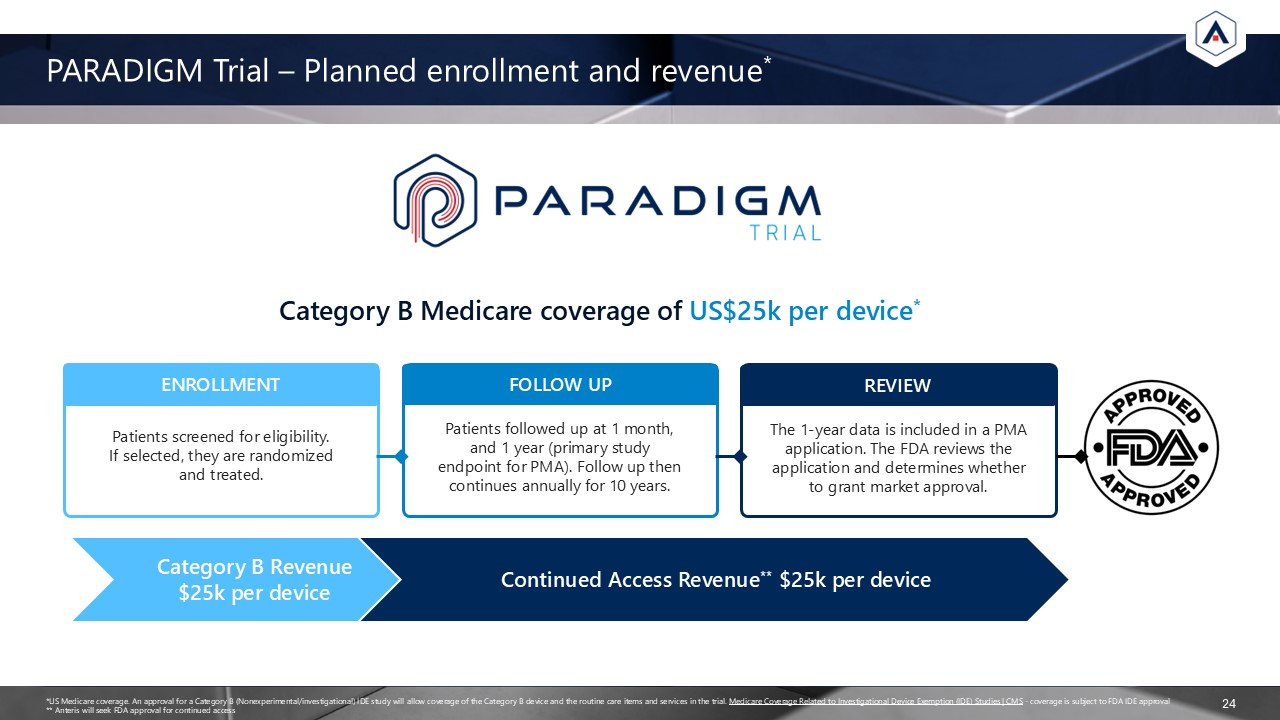
PARADIGM Trial – Planned enrollment and revenue* 24 Category B Medicare coverage
of US$25k per device* ENROLLMENT Patients screened for eligibility. If selected, they are randomized and treated. FOLLOW UP Patients followed up at 1 month, and 1 year (primary study endpoint for PMA). Follow up then continues annually for
10 years. REVIEW The 1-year data is included in a PMA application. The FDA reviews the application and determines whether to grant market approval. *US Medicare coverage. An approval for a Category B (Nonexperimental/investigational) IDE
study will allow coverage of the Category B device and the routine care items and services in the trial. Medicare Coverage Related to Investigational Device Exemption (IDE) Studies | CMS - coverage is subject to FDA IDE approval ** Anteris
will seek FDA approval for continued access
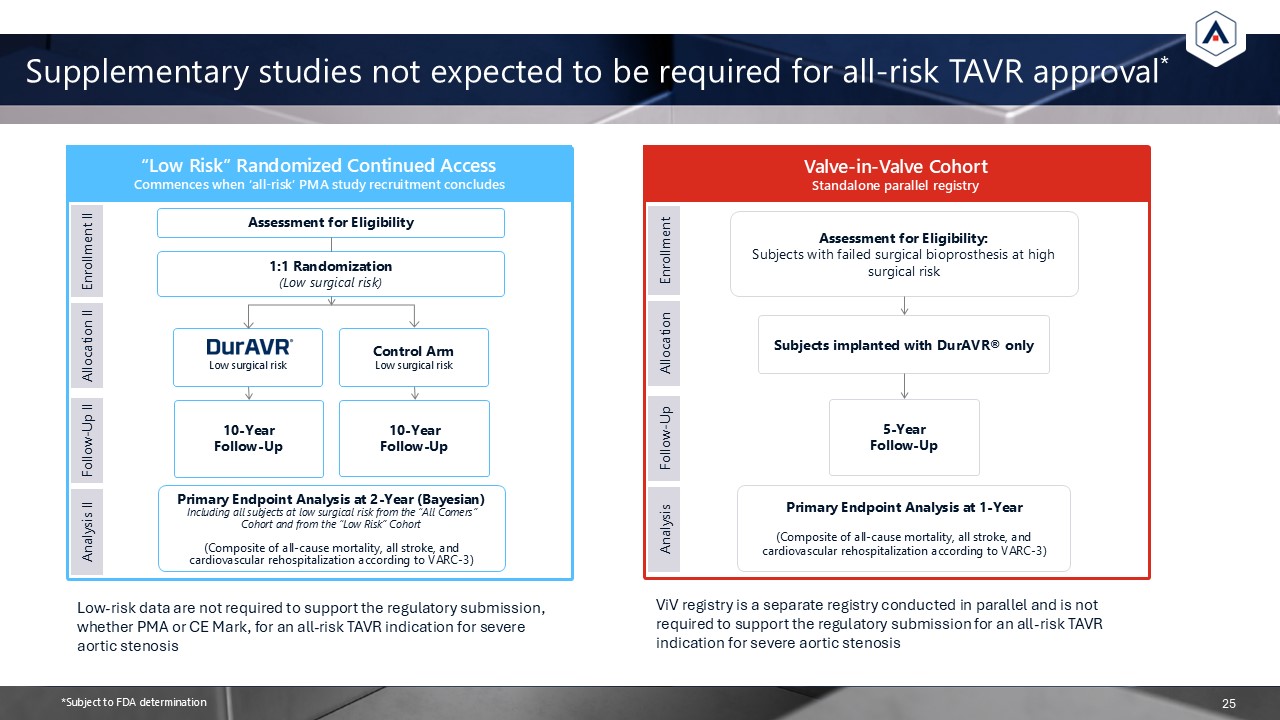
Supplementary studies not expected to be required for all-risk TAVR
approval* “Low Risk” Randomized Continued Access Commences when ‘all-risk’ PMA study recruitment concludes Enrollment II Allocation II Follow-Up II Analysis II Assessment for Eligibility 1:1 Randomization(Low surgical risk) Low
surgical risk Control ArmLow surgical risk 10-YearFollow-Up 10-YearFollow-Up Primary Endpoint Analysis at 2-Year (Bayesian) Including all subjects at low surgical risk from the “All Comers” Cohort and from the “Low Risk” Cohort(Composite
of all-cause mortality, all stroke, and cardiovascular rehospitalization according to VARC-3) Valve-in-Valve Cohort Standalone parallel registry Enrollment Allocation Follow-Up Analysis Assessment for Eligibility:Subjects with failed
surgical bioprosthesis at high surgical risk Subjects implanted with DurAVR® only 5-YearFollow-Up Primary Endpoint Analysis at 1-Year(Composite of all-cause mortality, all stroke, and cardiovascular rehospitalization according to
VARC-3) 25 Low-risk data are not required to support the regulatory submission, whether PMA or CE Mark, for an all-risk TAVR indication for severe aortic stenosis ViV registry is a separate registry conducted in parallel and is not required
to support the regulatory submission for an all-risk TAVR indication for severe aortic stenosis *Subject to FDA determination
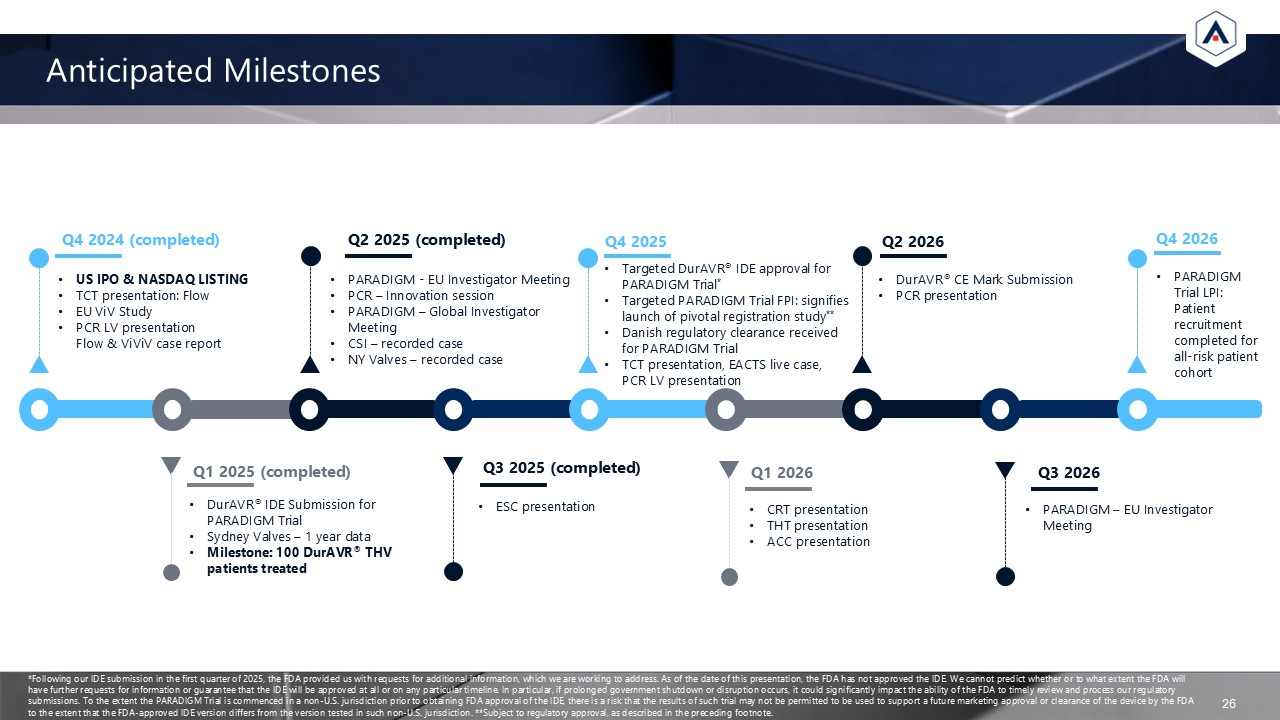
Anticipated Milestones 26 US IPO & NASDAQ LISTING TCT presentation: Flow
EU ViV Study PCR LV presentationFlow & ViViV case report Q4 2024 (completed) PARADIGM - EU Investigator Meeting PCR – Innovation session PARADIGM – Global Investigator Meeting CSI – recorded case NY Valves – recorded case Q2
2025 (completed) DurAVR® IDE Submission for PARADIGM Trial Sydney Valves – 1 year data Milestone: 100 DurAVR® THV patients treated Q1 2025 (completed) ESC presentation Q3 2025 (completed) Targeted DurAVR® IDE approval for PARADIGM
Trial* Targeted PARADIGM Trial FPI: signifies launch of pivotal registration study** Danish regulatory clearance receivedfor PARADIGM Trial TCT presentation, EACTS live case, PCR LV presentation Q4 2025 DurAVR® CE Mark Submission PCR
presentation Q2 2026 CRT presentation THT presentation ACC presentation Q1 2026 Q3 2026 PARADIGM Trial LPI: Patient recruitment completed for all-risk patientcohort Q4 2026 PARADIGM – EU Investigator Meeting *Following our IDE
submission in the first quarter of 2025, the FDA provided us with requests for additional information, which we are working to address. As of the date of this presentation, the FDA has not approved the IDE. We cannot predict whether or to what
extent the FDA will have further requests for information or guarantee that the IDE will be approved at all or on any particular timeline. In particular, if prolonged government shutdown or disruption occurs, it could significantly impact the
ability of the FDA to timely review and process our regulatory submissions. To the extent the PARADIGM Trial is commenced in a non-U.S. jurisdiction prior to obtaining FDA approval of the IDE, there is a risk that the results of such trial may
not be permitted to be used to support a future marketing approval or clearance of the device by the FDA to the extent that the FDA-approved IDE version differs from the version tested in such non-U.S. jurisdiction. **Subject to regulatory
approval, as described in the preceding footnote.

Key Takeaways 27 1 DurAVR® THV - Proprietary, new class of TAVR for aortic
stenosis - Easy, predictable balloon expandable deployment with the function of a healthy, native aortic valve Building commercial readiness for a new class of TAVR that mimics a healthy aortic valve Future Market Insights. Transcatheter
Heart Valve Replacement (TAVR) Market: Global Industry Analysis 2016 – 2023 and Opportunity Assessment 2024 – 2034. Future Market Insights; 2024. Available from:
https://www.futuremarketinsights.com/reports/transcatheter-heart-valve-replacement-tavi-market. *Subject to regulatory approval US$9.9bn global TAVR market forecasted by 20281 with many untreated patients- DurAVR® was designed to offer
advantages over two TAVR market leaders 2 Clinically validated with 130 patients- 30 day and 1 year data supports strong DurAVR® safety profile and hemodynamics 3 PARADIGM Trial targeted start 4Q 2025* potentially supporting FDA & CE
Mark filings- High quality global KOL adoption expected to drive rapid trial enrollment 4 Commercial ready - capital efficient go to market plan- Highly experienced clinical & commercial leadership plus infrastructure in place 5

Appendices

Anteris is guided by a global team of well-regarded cardiovascular Physician
advisors 29 Medical Advisory Board North America Europe Australia
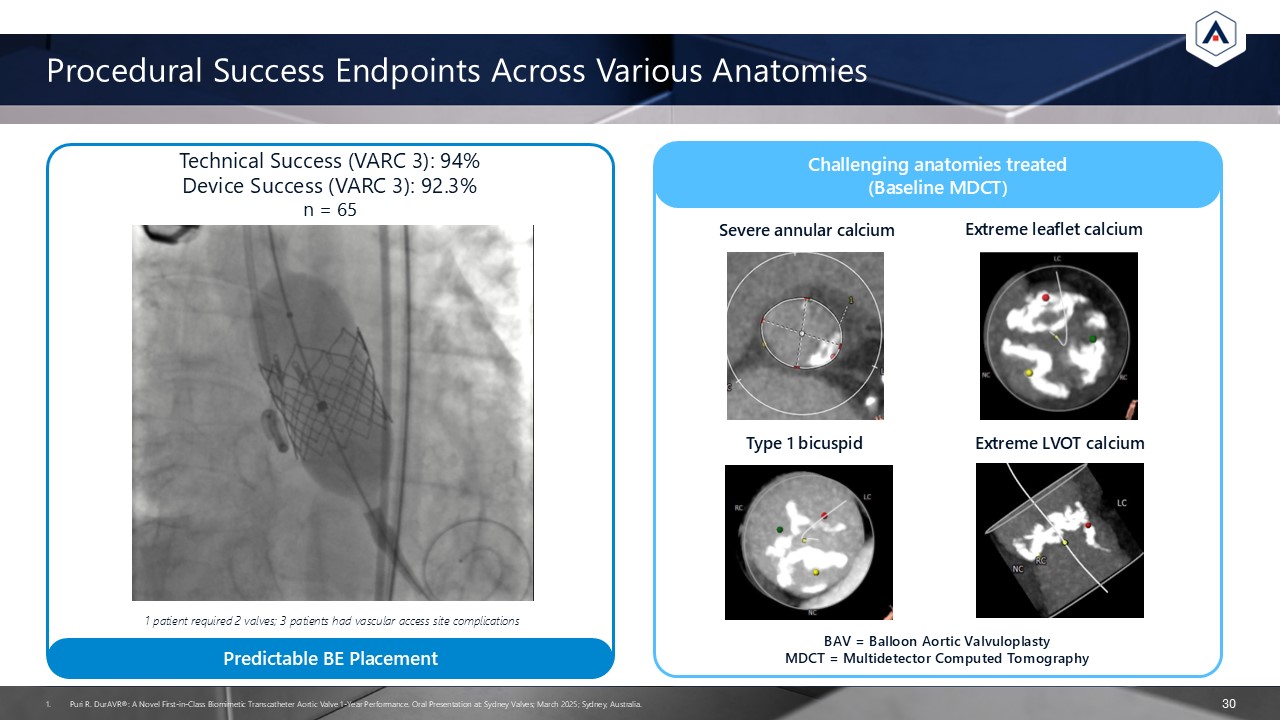
Technical Success (VARC 3): 94%Device Success (VARC 3): 92.3%n = 65 Procedural
Success Endpoints Across Various Anatomies 30 1 patient required 2 valves; 3 patients had vascular access site complications Severe annular calcium Extreme leaflet calcium Type 1 bicuspid Extreme LVOT calcium BAV = Balloon Aortic
Valvuloplasty MDCT = Multidetector Computed Tomography Predictable BE Placement Challenging anatomies treated (Baseline MDCT) Puri R. DurAVR®: A Novel First-in-Class Biomimetic Transcatheter Aortic Valve 1-Year Performance. Oral
Presentation at: Sydney Valves; March 2025; Sydney, Australia.
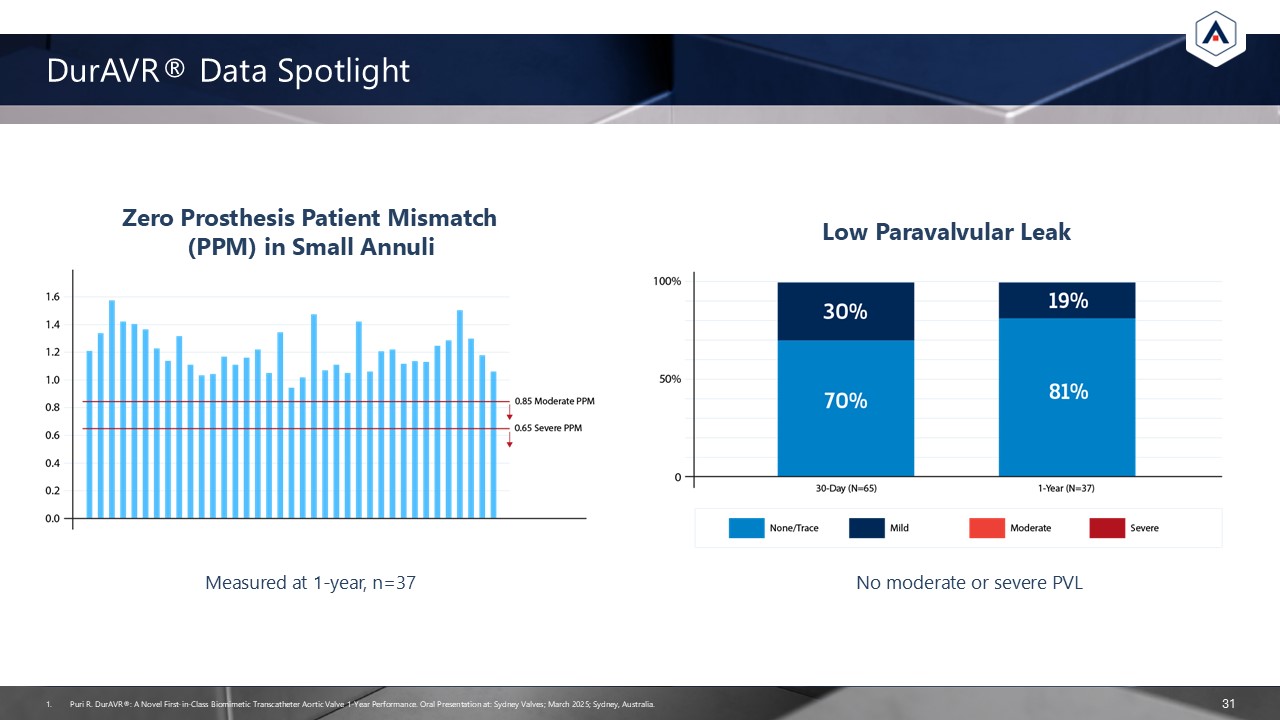
DurAVR® Data Spotlight 31 Zero Prosthesis Patient Mismatch (PPM) in Small
Annuli Low Paravalvular Leak Measured at 1-year, n=37 No moderate or severe PVL Puri R. DurAVR®: A Novel First-in-Class Biomimetic Transcatheter Aortic Valve 1-Year Performance. Oral Presentation at: Sydney Valves; March 2025; Sydney,
Australia.